Limitations of Diluting Citrate-Stabilized Gold Nanoparticles | Nanopartz™
Gold nanoparticles (AuNPs) are widely used in various applications, including biomedical research, sensing, and catalysis, due to their unique optical, electronic, and chemical properties. One common method of stabilizing gold nanoparticles is through adsorption of citrate ligands. Citrate-stabilized gold nanoparticles are popular because citrate ions provide a negative charge to the nanoparticle surface, creating an electrostatic repulsion that prevents aggregation. However, diluting these nanoparticles can lead to significant challenges.
Understanding Citrate Stabilization
Citrate ions adsorb onto the surface of gold nanoparticles, forming a negatively charged layer that stabilizes the particles in a colloidal solution. This negative charge creates an electrostatic repulsion between individual nanoparticles, preventing them from coming too close to each other and aggregating.
Challenges and Limitations of Dilution
When citrate-stabilized gold nanoparticles are diluted, several issues can arise that compromise their stability:
- Reduced Electrostatic Repulsion:
- Effect of Dilution: Dilution decreases the concentration of citrate ions in the solution, which weakens the electrostatic repulsion between nanoparticles.
- Outcome: As the repulsion decreases, nanoparticles are more likely to come into close contact, leading to aggregation.
- Decreased Colloidal Stability:
- Effect of Dilution: The stability of colloidal gold nanoparticles depends on a delicate balance between attractive van der Waals forces and repulsive electrostatic forces. Dilution disrupts this balance.
- Outcome: In a diluted solution, the reduced concentration of stabilizing ions can lead to nanoparticle instability, causing them to clump together and precipitate out of the solution.
- pH and Ionic Strength Sensitivity:
- Effect of Dilution: Dilution can also alter the pH and ionic strength of the solution. Since citrate-stabilized nanoparticles are sensitive to changes in pH and ionic strength, dilution can lead to destabilization.
- Outcome: This sensitivity can cause nanoparticles to aggregate more readily in diluted conditions, especially if the pH moves away from the optimal range for stability.
- Aggregation and Loss of Functionality:
- Effect of Dilution: Aggregation of gold nanoparticles due to dilution reduces their effective surface area and changes their optical properties.
- Outcome: This aggregation can lead to a loss of the unique functionalities of the nanoparticles, such as their ability to catalyze reactions or their optical response in sensing applications.
Practical Considerations
When working with citrate-stabilized gold nanoparticles, it's crucial to consider the implications of dilution:
- Avoiding Over-Dilution: To maintain stability, it's important to avoid excessive dilution. If dilution is necessary, it may be beneficial to add additional stabilizing agents or adjust the pH to maintain the electrostatic repulsion.
- Alternative Stabilization Methods: For applications requiring significant dilution, consider using alternative stabilization methods such as PEGylation, which provides steric stabilization and can be more robust under diluted conditions.
Visual Representation
Below is an image illustrating the behavior of citrate-stabilized gold nanoparticles in a colloidal solution and what happens upon dilution:
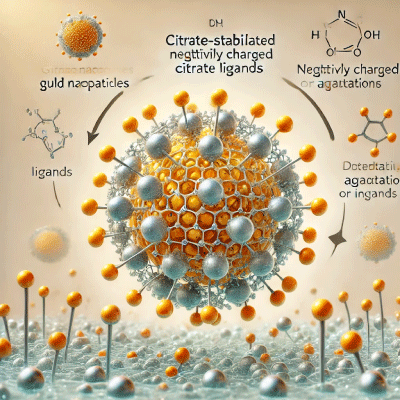
This image demonstrates how the citrate ligands create a negative charge around the nanoparticles, preventing aggregation. Upon dilution, the decreased concentration of citrate ions leads to reduced stability and potential aggregation.
Conclusion
While citrate-stabilized gold nanoparticles offer effective stabilization under certain conditions, dilution can significantly compromise their stability, leading to aggregation and loss of functionality. Careful consideration of the factors discussed above is essential when using these nanoparticles in diluted solutions.
For more information and to explore their products, visit the Nanopartz™ website. Happy researching!
Go here for Nanopartz Gold Nanoparticles

