Antibody Conjugation to Gold Nanoparticles: Adsorption vs. Covalent Bridge Attachment
Antibody conjugation to gold nanoparticles (AuNPs) is a critical process in biomedical applications such as diagnostics, imaging, and therapeutics. The conjugation method significantly impacts nanoparticle stability, bioactivity, and assay performance. The two primary methods of conjugation are adsorption and covalent bridge attachment.
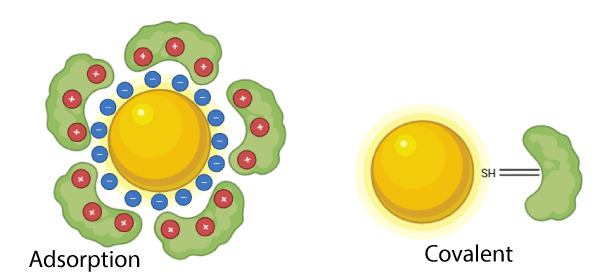
Adsorption vs. Covalent Bridge Attachment
Adsorption
Adsorption-based conjugation relies on the natural affinity of antibodies for the gold nanoparticle surface, primarily through cysteine residues, which contain thiol (-SH) groups. Thiol groups have a strong affinity for gold, forming a gold-thiol bond, which is often considered a quasi-covalent interaction rather than purely non-covalent.
Advantages
- Simple and rapid process.
- No need for chemical modification of antibodies.
- Strong gold-thiol interactions provide moderate stability.
Disadvantages
- Random antibody orientation, which can reduce antigen-binding efficiency.
- Surface competition with other biomolecules in complex biological environments.
- Weaker than engineered covalent bonding, leading to potential desorption over time.
Covalent Bridge Attachment
Covalent conjugation involves chemically linking the antibody to the gold nanoparticle using a molecular bridge, ensuring a stronger and more stable attachment with controlled orientation.
Advantages
- Strong, irreversible attachment prevents desorption.
- Higher stability in diverse physiological conditions.
- Controlled orientation enhances antigen recognition and bioactivity.
Disadvantages
- Requires chemical modifications of antibodies.
- More complex conjugation process.
- Longer bridges may impact bioactivity and nanoparticle performance.
Impact of Bridge Length in Covalent Conjugation
The length of the covalent linker influences the stability, accessibility, and performance of the conjugated antibody.
- Short Linkers: Ensure close proximity of the antibody to the nanoparticle surface, which enhances stability and improves sensitivity to analytes. However, this often comes at the cost of increased non-specific binding, as the reduced spatial separation may lead to unintended interactions with surrounding molecules.
- Medium-Length Linkers: Provide a balance between stability, sensitivity, and specificity, making them ideal for most applications.
- Long Linkers: Increase antibody flexibility and antigen access, reducing non-specific binding but potentially leading to lower sensitivity due to increased distance from the nanoparticle surface.
Types of Covalent Bridge Attachments
- Carbodiimide (EDC/NHS) Crosslinking – Activates carboxyl groups on the antibody or nanoparticle for stable amide bond formation.
- Maleimide-Thiol Chemistry – Targets thiol groups on antibodies for site-specific conjugation.
- Click Chemistry (Azide-Alkyne Cycloaddition) – Provides highly selective and bioorthogonal conjugation.
- PEGylation – Involves polyethylene glycol (PEG) spacers to enhance stability and bioavailability.
Covalent Bridge Polymers for Antibody Conjugation
Various polymeric linkers are used for covalent antibody conjugation, each offering unique properties in terms of flexibility, hydrophilicity, and biocompatibility.
- Polyethylene Glycol (PEG):
- Enhances solubility and reduces aggregation.
- Minimizes non-specific interactions.
- Available in various lengths to fine-tune antigen accessibility.
- Alkanethiols:
- Forms strong and stable thiol-gold bonds.
- Allows for precise control over linker length and orientation.
- Can introduce hydrophobic or hydrophilic properties depending on the functional groups.
- Dextran and Polysaccharides:
- Biocompatible and hydrophilic, reducing non-specific binding.
- Commonly used for passivation and stabilization of conjugates.
- Polypeptides (e.g., Poly-Lysine):
- Can provide multiple attachment sites for antibodies.
- Facilitates controlled assembly of biomolecules on the nanoparticle surface.
- Polyvinylpyrrolidone (PVP):
- Provides excellent colloidal stability.
- Reduces opsonization and immune recognition, making it useful for in vivo applications.
Conclusion
While antibody adsorption via thiol groups (gold-thiol interaction) is strong and convenient, it lacks control over orientation and long-term stability. Covalent bridge attachment, using functionalized linkers, ensures precise antibody positioning and higher stability, making it the preferred method for applications requiring optimal performance. The choice of linker length plays a crucial role in balancing sensitivity and specificity, with shorter linkers enhancing sensitivity at the expense of non-specific binding, while longer linkers reduce non-specific interactions but may slightly lower detection sensitivity. The selection of polymeric linkers, such as PEG, alkanethiols, and polysaccharides, further optimizes stability, bioactivity, and performance for specific applications.

