Tail Vein and Oral Injection of Gold Nanoparticle Solutions in Mice: Protocols, Methods, and Best Practices
Learn the detailed protocols for tail vein and oral injection of gold nanoparticle (AuNP) solutions in mice, including step-by-step procedures, best practices, troubleshooting, and safety considerations for researchers.
Disclaimer: This technical note should not be used a sole source for information on this topic. It is intended as an overview of current articles available in literature and should only be used as a starting point for the researcher.
Table of Contents
Tail Vein Injection Procedure for Gold Nanoparticle Solutions
Oral Gavage (Oral Injection) of Gold Nanoparticle Solutions in Mice
Safety Considerations and Ethical Approvals for Animal Studies
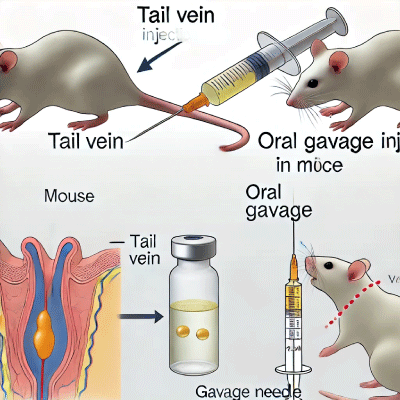
Here are two detailed scientific diagrams illustrating both the tail vein and oral gavage injection procedures in mice. These visuals can complement your webpage on administering gold nanoparticle solutions in mice.
Tail Vein Injection Procedure for Gold Nanoparticle Solutions
Overview
Tail vein injection is a widely used method for intravenous administration of various solutions in mice, including nanoparticles. Gold nanoparticles (AuNPs) have unique physicochemical properties, making them attractive for drug delivery, imaging, and therapeutic applications in preclinical research. This technical note outlines the protocol for administering gold nanoparticle solutions via tail vein injection in mice. The procedure is minimally invasive, allowing for precise systemic delivery with rapid distribution through the circulatory system.
Materials and Equipment
- Gold nanoparticles (AuNPs) solution: Ensure appropriate size and concentration of nanoparticles (typically 5-50 nm in size).
- Syringes: 1 mL insulin syringes (27-30G needle).
- 70% ethanol for disinfection.
- Heat lamp or warm water bath to dilate the tail vein.
- Restraint device or manual handling techniques for mouse immobilization.
- Anesthetic agents (optional): Isoflurane or injectable anesthesia if sedation is necessary.
- Sterile gauze and cotton swabs for clean-up.
Procedure
-
Preparation of the Mouse:
- Warm the mouse tail using a heat lamp or warm water bath for 1-2 minutes to dilate the tail veins, making them more visible and accessible. Ensure the mouse is not overheated or stressed.
- Clean the tail with a cotton swab soaked in 70% ethanol to disinfect the injection site and improve vein visibility.
- Preparation of the AuNPs Solution:
- Ensure the gold nanoparticles solution is sterile and well-dispersed. The solution should be free from any aggregates, as these could obstruct the needle or cause blockages in blood vessels. Sonication may be used to disperse nanoparticles before injection.
- Withdraw the desired volume of AuNPs solution (usually 100-200 µL, depending on the experimental design) into a 1 mL syringe equipped with a 27-30G needle.
- Dilute the gold nanoparticle solution with sterilized saline. Typical injection concentrations are 2.5ug per 10g body weight in 1mL saline.
- Tail Vein Injection:
- Identify the lateral tail vein. It appears as a blue or dark line running along either side of the tail.
- Insert the needle at a shallow angle (10-15°) into the vein. The needle should be bevel up to ensure proper vein penetration.
- Once inside the vein, gently aspirate to confirm proper placement (a small amount of blood should enter the syringe).
- Slowly inject the AuNPs solution into the vein. The injection should take around 5-10 seconds depending on the volume. If resistance is felt, stop the injection to prevent extravasation.
- After completing the injection, withdraw the needle slowly and apply gentle pressure to the injection site with sterile gauze to prevent bleeding.
- Post-Injection Monitoring:
- Monitor the mouse for any signs of distress or abnormal behavior, such as labored breathing or reduced mobility. Ensure the animal recovers fully if anesthesia was used.
- Return the mouse to its cage and observe for at least 10-15 minutes to ensure no adverse reactions.
Troubleshooting
- Failed vein access: If blood cannot be drawn or there is significant resistance during injection, the needle may not be correctly positioned in the vein. Gently reposition the needle or use an alternative vein (opposite tail vein).
- Extravasation: If the solution leaks outside the vein, swelling or discoloration will occur at the injection site. Halt the injection immediately and apply a cold compress. Select another injection site if further injections are needed.
Safety Considerations
- Handle all needles and syringes with care to avoid accidental injuries. Dispose of them in designated sharps containers.
- Work in a sterile environment to prevent contamination of the AuNPs solution or infection at the injection site.
Notes on Nanoparticle Characteristics
-
The biodistribution, pharmacokinetics, and clearance of gold nanoparticles are highly dependent on their size, shape, surface charge, and functionalization. For instance, smaller nanoparticles (5-20 nm) are often cleared rapidly via renal pathways, while larger nanoparticles (>20 nm) may be retained longer in circulation and cleared via the reticuloendothelial system (RES) .
-
Surface modifications, such as polyethylene glycol (PEG) coating, can increase circulation time by reducing opsonization and subsequent clearance by the RES .
Conclusion
Tail vein injection of gold nanoparticles is a reliable method for systemic administration in mice, offering efficient nanoparticle delivery to various organs and tissues. This method requires attention to technique and nanoparticle characterization to ensure reproducibility and safety.
References
- Albanese, A., Tang, P. S., & Chan, W. C. W. (2012). The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annual Review of Biomedical Engineering, 14, 1–16.
- Wang, Y., Grainger, D. W. (2013). Nanoparticle biodistribution, clearance, and toxicity: Considerations in the design of nanotherapeutics. Advanced Drug Delivery Reviews, 65(1), 63-71.
- Gref, R., Luck, M., Quellec, P., et al. (2000). 'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids and Surfaces B: Biointerfaces, 18(3-4), 301-313.
Oral Gavage (Oral Injection) of Gold Nanoparticle Solutions in Mice
Overview
Oral gavage is a common method used to administer substances directly into the stomach of mice, bypassing the need for food or water intake. This technique is particularly useful for delivering precise doses of solutions, such as gold nanoparticles (AuNPs), which are used in various biomedical applications, including drug delivery, imaging, and diagnostics. Gold nanoparticles administered via oral gavage can provide valuable information about gastrointestinal absorption, bioavailability, biodistribution, and potential toxicity.
This technical note outlines the procedure for administering a gold nanoparticle solution via oral gavage in mice and highlights important considerations for ensuring animal welfare and accurate experimental results.
Materials and Equipment
- Gold nanoparticles (AuNPs) solution: Ensure the solution is sterile and well-dispersed. The size of the nanoparticles should be consistent with the study design (typically 5-50 nm).
- Gavage needle: A curved, ball-tipped gavage needle made of stainless steel or plastic (20-22G for adult mice, 22-24G for younger or smaller mice).
- Syringes: 1 mL syringe for drawing the AuNPs solution and attaching to the gavage needle.
- Restraint device or manual technique to immobilize the mouse.
- 70% ethanol for disinfection.
- Scale for weighing mice to ensure correct dosing based on body weight.
- Sterile gauze and cotton swabs for cleaning.
Procedure
-
Preparation of the Mouse:
- Weigh the mouse to determine the correct dose of the gold nanoparticle solution. Dosing is typically based on the body weight of the mouse, with volumes not exceeding 10 mL/kg of body weight.
- Restrain the mouse securely using a restraint device or by manual scruffing. Proper restraint is essential to minimize movement and reduce stress during the gavage procedure.
- Preparation of the AuNPs Solution:
- Ensure the gold nanoparticles solution is sterile and well-dispersed. The solution should be free from any aggregates, as these could obstruct the needle or cause blockages in blood vessels. Sonication may be used to disperse nanoparticles before injection.
- Withdraw the desired volume of AuNPs solution (usually 100-200 µL, depending on the experimental design) into a 1 mL syringe equipped with a 27-30G needle.
- Dilute the gold nanoparticle solution with sterilized saline. Typical injection concentrations are 2.5ug per 10g body weight in 1mL saline.
- Gavage Administration:
- Gently tilt the mouse’s head upwards to align the mouth with the esophagus.
- Insert the gavage needle into the mouth and guide it along the roof of the mouth until you feel it slip down the esophagus. Avoid forcing the needle, as this can cause injury to the trachea or esophagus.
- Once the gavage needle is positioned correctly in the esophagus, slowly inject the AuNPs solution into the stomach. The entire injection should take about 5-10 seconds to ensure the liquid is properly delivered and absorbed.
- After the injection, carefully withdraw the needle and observe the mouse to ensure it exhibits no signs of distress.
- Post-Injection Monitoring:
- After administration, monitor the mouse for at least 15-30 minutes to check for any adverse effects such as difficulty breathing, coughing, or lethargy, which may indicate improper gavage (e.g., accidental tracheal insertion).
- Ensure that the mouse returns to normal behavior (e.g., grooming, exploring) before placing it back into the cage.
Troubleshooting
-
Difficulty inserting the needle: If the needle does not pass easily, it may be in the trachea. In such cases, withdraw the needle and reposition it. Ensure that the mouse’s head is properly aligned with the body to allow smooth passage.
-
Aspiration of liquid: If the mouse exhibits signs of distress (e.g., coughing, difficulty breathing), stop the procedure immediately. Aspiration of the AuNPs solution into the lungs can lead to pneumonia or death. In such cases, euthanasia may be necessary if the animal is suffering.
-
Regurgitation: If the mouse regurgitates the solution, it may indicate that the volume is too large or that the solution was administered too quickly. Reduce the volume and administer the solution more slowly in future attempts.
Considerations for Nanoparticle Administration
- Nanoparticle Characterization: Gold nanoparticles used for oral gavage should be well-characterized in terms of size, shape, surface charge, and coating (e.g., polyethylene glycol, PEG). These factors influence gastrointestinal absorption, biodistribution, and clearance.
- Stability in the Gastrointestinal Tract: The gastric environment can affect the stability and bioavailability of nanoparticles. Surface coatings (e.g., PEGylation) can improve the stability of gold nanoparticles in the stomach and enhance their absorption in the intestines.
- Dosing Considerations: Gold nanoparticle toxicity is dose-dependent. Researchers should carefully determine the appropriate dose based on the particle size, surface chemistry, and animal’s body weight. Lower doses should be tested first to establish safety before scaling up.
Safety Considerations
- Minimizing Stress: Proper handling and restraint are essential to minimize stress to the mouse. Excessive stress can alter the experimental outcomes, including physiological responses to nanoparticle administration.
- Equipment Sterilization: All gavage needles, syringes, and equipment should be sterilized before use to prevent infections.
- Euthanasia: If gavage results in aspiration or severe complications, euthanasia may be required following institutional guidelines, such as those provided by the American Veterinary Medical Association (AVMA) guidelines for humane euthanasia.
Notes on Data Interpretation
- Bioavailability of Nanoparticles: Nanoparticles administered orally face different physiological barriers compared to intravenous injection. The nanoparticles must pass through the stomach and intestines, where they can undergo chemical changes, degradation, or adsorption by intestinal cells. This could reduce the bioavailability of gold nanoparticles compared to other administration routes.
- Tissue Distribution: The biodistribution of orally administered gold nanoparticles may vary, with particles potentially accumulating in organs such as the liver, spleen, kidneys, and intestines. Researchers should plan to perform tissue collection and analysis to study the biodistribution.
Conclusion
Oral gavage is a valuable technique for administering gold nanoparticles to mice, allowing for precise dosing and control over delivery. When performed correctly, oral gavage enables researchers to investigate the gastrointestinal absorption, biodistribution, and effects of nanoparticles on various biological systems.
References
- De Jong, W. H., & Borm, P. J. (2008). Drug delivery and nanoparticles: Applications and hazards. International Journal of Nanomedicine, 3(2), 133-149.
- Kim, J., Lee, S., & Park, K. (2017). Gold nanoparticle-based drug delivery systems for tumor targeting. Journal of Controlled Release, 246, 276-284.
- Smith, D. M., Simon, J. K., & Baker, J. R. (2013). Applications of nanotechnology for immunology. Nature Reviews Immunology, 13(8), 592-605.
- Ma, Z., & Lim, T. M. (2016). Understanding the bioavailability of nanoparticles through oral gavage and its physiological significance. Journal of Drug Delivery Science and Technology, 35, 105-113.
- American Veterinary Medical Association (2020). AVMA Guidelines for the Euthanasia of Animals. Available at: https://www.avma.org
This technical note provides guidance for researchers to administer gold nanoparticle solutions via oral gavage in mice, ensuring accuracy, safety, and ethical compliance in experimental research.
Safety Considerations and Ethical Approvals for Animal Studies
Working with mice in a university environment typically requires several layers of approval to ensure ethical, legal, and humane treatment of the animals. These approvals vary slightly depending on the country and institution, but the general requirements include:
1. Institutional Animal Care and Use Committee (IACUC) Approval
- IACUC is the primary body responsible for overseeing all animal research at institutions in the U.S. and many other countries. Every project involving the use of vertebrate animals must be reviewed and approved by the IACUC before the research can begin.
- Protocol Submission: Researchers must submit a detailed animal use protocol that includes the rationale for using animals, the number of animals required, species, experimental procedures, anesthesia and euthanasia methods, and how animal suffering will be minimized.
- Ethical Justification: Researchers must justify why animals are necessary, why alternatives (e.g., in vitro studies or computer models) cannot be used, and ensure that the proposed research adheres to the principles of the "3 Rs" (Replacement, Reduction, Refinement):
- Replacement: Using alternatives to animals when possible.
- Reduction: Using the fewest number of animals necessary to achieve the research goals.
- Refinement: Modifying procedures to minimize pain, distress, or suffering to the animals.
2. Institutional Biosafety Committee (IBC) Approval (if applicable)
- If the research involves genetically modified organisms (GMOs), pathogens, or hazardous biological materials, researchers may also need approval from the Institutional Biosafety Committee (IBC).
- This committee ensures that experiments involving recombinant DNA (rDNA), infectious agents, or biological toxins are conducted safely and in compliance with regulations, such as the National Institutes of Health (NIH) Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules.
3. Environmental Health and Safety (EHS) Approval (if applicable)
- If the research involves hazardous chemicals, radioactive materials, or any agents that may pose environmental or health risks, Environmental Health and Safety (EHS) approval is required.
- EHS ensures that proper safety protocols are in place, including safe handling, storage, and disposal of hazardous materials, and that lab personnel are adequately trained in safety procedures.
4. Veterinary Review
- In many cases, a university’s veterinary staff or attending veterinarian reviews the animal use protocol. They provide guidance on proper animal care, anesthesia, surgery, pain management, and euthanasia procedures.
- They may also provide direct support during the experiments, ensuring compliance with animal welfare standards and offering emergency care for animals if necessary.
5. Training and Certification for Personnel
- All personnel involved in the care, handling, or experimentation on animals must undergo training to ensure they are competent in animal welfare, handling techniques, and the specific procedures being performed.
- Training typically covers areas such as:
- Animal handling and restraint.
- Recognizing pain and distress in animals.
- Anesthesia, surgery, and post-operative care.
- Euthanasia procedures.
- Universities often require researchers and technicians to complete courses on regulatory guidelines, such as the Guide for the Care and Use of Laboratory Animals, which is a key resource on humane animal care.
6. State and Federal Regulations
- Depending on the location of the university, researchers must comply with local, state, and federal regulations that govern animal research.
- In the United States, relevant regulations include:
- Animal Welfare Act (AWA): Administered by the United States Department of Agriculture (USDA), the AWA sets minimum standards for the care and use of certain animals in research (though mice and rats are not covered by the AWA).
- Public Health Service (PHS) Policy: Enforced by the Office of Laboratory Animal Welfare (OLAW), this policy applies to all vertebrate animals (including mice and rats) used in PHS-funded research, ensuring compliance with standards outlined in the Guide for the Care and Use of Laboratory Animals.
- Food and Drug Administration (FDA) regulations may also apply if the research involves drug or device testing in animals.
7. USDA Animal Research Registration and Inspection
- While mice and rats are not covered under the USDA Animal Welfare Act, other vertebrate species are. If any such animals are used, universities must be registered with the USDA as a research facility and are subject to unannounced inspections to ensure compliance with the AWA.
8. Animal Care Accreditation (AAALAC)
- Many institutions seek voluntary accreditation through the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). This accreditation signifies that the institution follows high standards of animal care and use beyond basic regulatory requirements.
- While AAALAC accreditation is not mandatory, it is often considered a mark of excellence in animal research programs and may be required by certain funding agencies.
9. Ethics Approval for Specific Experimental Procedures
- Certain types of experiments, such as those involving significant pain, distress, or surgical interventions, may require additional ethical justification and review by the IACUC.
- If euthanasia is required, the method used must be in line with American Veterinary Medical Association (AVMA) guidelines for humane euthanasia.
Summary of Key Approvals Required
- IACUC approval for all animal research protocols.
- IBC approval if working with hazardous biological materials.
- EHS approval for hazardous chemicals or radiation.
- Training certification for all personnel handling animals.
- Compliance with state, federal, and institutional animal research laws and regulations.
Additional Considerations
Researchers should be prepared for ongoing oversight, such as:
- Annual protocol renewals and amendments.
- Regular facility inspections.
- Unannounced audits by regulatory bodies (e.g., USDA).
By following these approvals and protocols, researchers ensure ethical compliance and the humane treatment of animals in their research.
References
- National Research Council (2011). Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press.
- Office of Laboratory Animal Welfare (OLAW) (2015). Public Health Service Policy on Humane Care and Use of Laboratory Animals. Available at: https://olaw.nih.gov
- United States Department of Agriculture (USDA). Animal Welfare Act and Animal Welfare Regulations. Available at: https://www.aphis.usda.gov/
- American Veterinary Medical Association (2020). AVMA Guidelines for the Euthanasia of Animals.
For more information and to explore their products, visit the Nanopartz™ website. Happy researching!
Go here for Nanopartz Gold Nanoparticles

